Abstract
Background: Anti-BCMA CAR T-cell therapy for RRMM is highly effective at inducing CR/sCR in heavily pretreated, triple-class and penta-refractory patients. However, despite notable striking initial activity, the durability of clinical responses has been a major issue and most patients eventually relapse/progress. In general, in MM different relapse/PD patterns have been recognized including insidious or asymptomatic disease, symptomatic disease along with increase in serum/urine monoclonal component/FLC-escape, extramedullary disease and transformed disease such as plasma cell leukemia or plasmablastic myeloma. The precise knowledge of relapse/PD patterns following anti-BCMA CAR T-cell therapy is lacking in the literature to generate evidence-based surveillance recommendations after such novel therapy. We sought to examine the patterns of relapse and clinical outcomes in patients with relapsed myeloma treated with autologous anti-BCMA therapy in our institution.
Methods: We conducted a retrospective analysis of MM patients treated at H. Lee Moffitt Cancer Center & Research Institute with anti-BCMA CAR T-cell therapy on a clinical trial or commercially with a follow up of ≥ 3 months. The International Myeloma Working Group (IMWG) criteria for disease response assessment in MM was used to grade treatment responses and assess relapse.
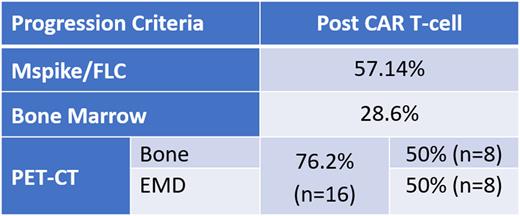
Results: At data cut off, a total of 87 patients have received BCMA-targeted CAR T-cell. Of those patients, 33 patients (37.9%) have relapsed following BCMA-targeted CAR T-cell and twenty-one patients were evaluable for analysis at the time of relapse. Patients excluded from the analysis include those with incomplete restaging (lack BM, lack PET-CT or MM labs), and those progressing before the 3-month evaluation post-CAR T-cell. The median number of prior lines of therapy was 4 ( range 1-10). ORR was 72.7% including sCR/CR (42.5%), VGPR (12.1%), PR (18.1%), SD (21.1%), and PD (6.1%). At the time of CAR T-cell, measurable disease as defined by IMWG consensus was present in 88% of patients and PET-CT was available in 24 patients at baseline and was positive for disease in 91.6%. All patients had baseline bone marrow (BM) assessment with 69.7% (n=23) showing involving by MM (PC ≥ 10%). Extramedullary disease (EMD) was present in 42.4% of patients at time of CAR T-cell. The median time to relapse was 5.29 months with median OS of 12.9 months from date of infusion. The median OS from relapse was 6.8 months. At relapse, 38% of patients met criteria for relapse only by PET-CT; and 23.8% met criteria only by Mspike/FLC assessment. Only 23% of patients met criteria for relapse by serological markers (Mspike/FLC), PET-CT, and BM. Of those evaluated by PET-CT at relapse, 76.2% (n=16) had evidence of relapse by the modality. At the time of relapse EMD was present in 38.1% of evaluable patients (table #1).
Conclusion: The prognosis of RRMM patients following relapse after anti-BCMA CAR T-cell therapy is dismal and standardized/evidence-based approaches for monitoring patient following such therapy are necessary. Our study demonstrates the need for a robust post-CAR T-cell monitoring strategy with the inclusion of PET-CT at different time points to intervene early in the course with personalized therapeutic strategies. Although we acknowledge the limitation of our study given its retrospective nature, we believe that our findings may strengthen the current clinical practice for monitoring patients with RRMM after BCMA-targeted CAR T-cell therapy and provide a drawing for upcoming studies.
Disclosures
Hansen:BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; Survivorship: Honoraria; OncLive: Honoraria. Grajales-Cruz:sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Blue:Oncopeptides: Honoraria; Jassen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Speakers Bureau. Freeman:Amgen: Honoraria; Sanofi: Honoraria; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria; Janssen: Honoraria, Research Funding. Shain:Takeda: Honoraria, Speakers Bureau; GlaxoSmithKline: Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Adaptive: Honoraria; Janssen: Honoraria, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Speakers Bureau; Karyopharm: Research Funding, Speakers Bureau; AbbVie: Research Funding. Liu:Sanofi: Speakers Bureau. Locke:Aptitude Health: Other: Education or editorial role; Leukemia and Lymphoma Society: Research Funding; Allogene: Membership on an entity's Board of Directors or advisory committees; Wugen: Membership on an entity's Board of Directors or advisory committees; Umoja: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Research Funding; Allogene: Research Funding; Novartis: Research Funding; BlueBird Bio: Research Funding; BMS: Research Funding; National Cancer Institute: Research Funding; Clinical Care Options Oncology: Other: Education or editorial role; Imedex: Other: Education or editorial role; Society for Immunotherapy of Cancer: Other: Education or editorial role; Takeda: Membership on an entity's Board of Directors or advisory committees; Sana: Membership on an entity's Board of Directors or advisory committees; Emerging Therapy Solutions: Consultancy; Gerson Lehrman Group: Consultancy; Moffitt Cancer Center: Patents & Royalties: several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy; BioPharma Communications CARE Education: Other: Education or editorial role; ASH: Other: Education or editorial role; Calibr: Membership on an entity's Board of Directors or advisory committees; Caribou: Membership on an entity's Board of Directors or advisory committees; Cellular Biomedicine Group: Membership on an entity's Board of Directors or advisory committees; Iovance: Membership on an entity's Board of Directors or advisory committees; GammaDelta Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Legend Biotech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Cowen: Consultancy; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; EcoR1: Consultancy; A2: Membership on an entity's Board of Directors or advisory committees. Baz:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; genentech: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; celgene: Consultancy, Honoraria; karyopharm: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Shattuck labs: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria; Merck: Research Funding. Alsina:BMS: Research Funding; BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal